Es geht nicht nur um Fakten… aber auch
Tierversuche sind sinnlos? Alles Quatsch? Ganz so einfach ist es nicht. Hier schauen wir uns deshalb ein paar Fakten rund um Versuchstiere genauer an. Fehlt etwas? Dann gebt uns gern Bescheid, und wir recherchieren es für Euch.

Frequently Asked Questions
Informationen über Tierversuche gibt es im Internet viele – aber nicht unbedingt gute. Hier greifen wir einige der häufigsten Fragen auf.

Faktencheck 1: die Basics
Antworten auf allgemeine Fragen zu Tierversuche – und zur Grundlagenforschung
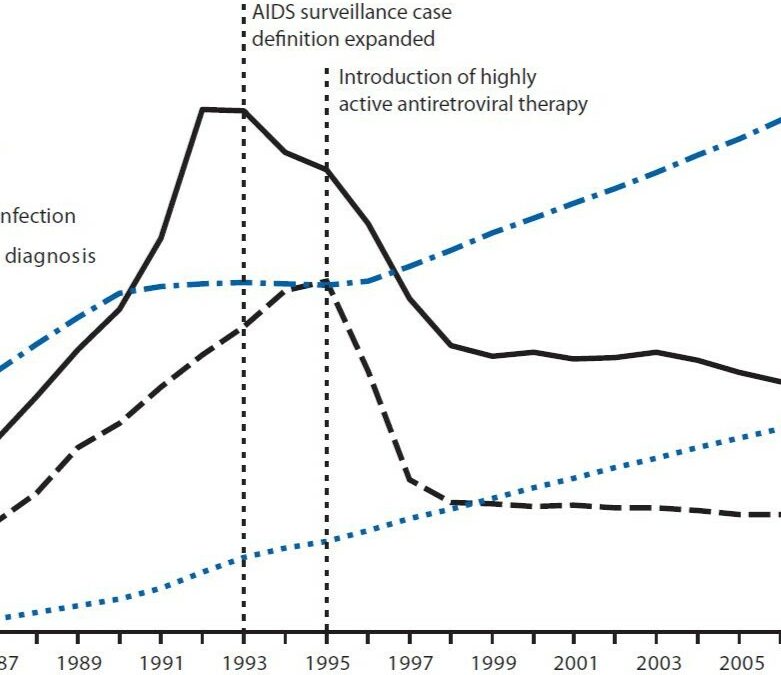
Faktencheck 2: rund um Medizin
Antworten auf Fragen rund um Krankheiten und Medikamente

Grundlagenforschung: Über „Neugier“ und ihre Folgen
Warum es „Neugierforschung“ gibt, und wie sie mit anwendungsorientierter Forschung zusammenhängt

Alternativen zu Tierversuchen
Es gibt schon viele tolle Alternativen und Ersatzverfahren für einige Tierversuche. Davon wollen wir euch natürlich auch erzählen.

Tiermodelle
Nicht erst seit Twitter-Accounts wie @justsaysinmice ist klar, wie oft Tiere als Modelle für Menschen bzw. menschliche Krankheiten dienen. Wir wollen euch gewöhnliche und ungewöhnliche Vertreter vorstellen!

Medizin-Nobelpreise
Über die Durchbrüche der letzten Jahre und Jahrzehnte… und was sie mit Tierversuchen zu tun haben.





